Weekly report: Pre-Production Product Briefing
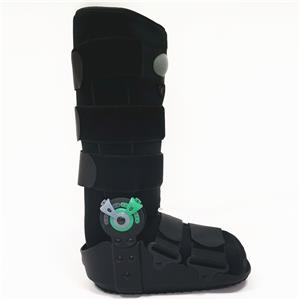
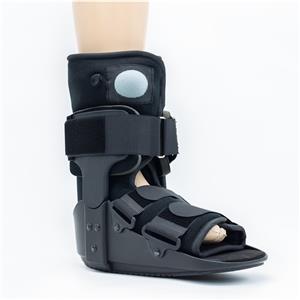
Weekly product presentations are held as scheduled. Today we are introducing several products that are about to go into mass production as knee pads. Since we provide customized service, some of the products we will modify according to the requirements of our guests, which is still some differences from our regular products. So before the product enters into mass production, we will surely gather all departments together to have a product introduction meeting. The purpose is to let everyone understand more clearly what details of the batch of products we are about to produce have been changed, and what places need to be paid attention to, in order to avoid the emergence of errors in large quantities.
We will not only pre-production of the product introduction of the propaganda, in the production process will also have quality inspection patrol the entire workshop, timely detection of production problems. After the production is completed, it needs to be inspected by the quality inspection, and can only be shipped after passing the inspection. A series of quality control, so that our products can be delivered to customers with high quality.
If you are interested in our company and products, please mail to sales4@huakangortho.com. We will provide you with competitive price and reliable quality. We maintain long-term cooperation with all our customers because we are aggressive, innovative and provide high quality goods.